Right Now
Global Wearable Sleep Apnea Detector Market to Grow at 8.8% CAGR Through 2031
Global Wearable Sleep Apnea Detector Market to Grow at 8.8% CAGR Through 2031
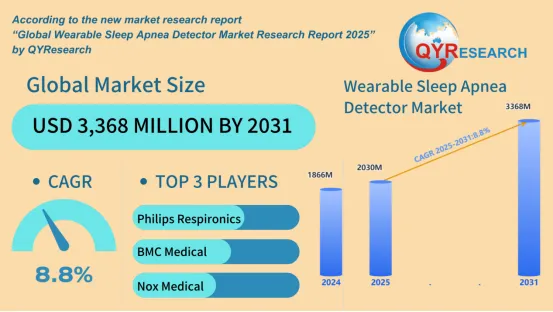
The global wearable sleep apnea detector market, valued at USD 1,866 million in 2024, is projected to reach USD 3,368 million by 2031, according to the Global Wearable Sleep Apnea Detector Market Research Report 2025 by QYResearch. With a CAGR of 8.8% forecasted during the 2025–2031 period, the market is being shaped by growing awareness of sleep disorders, increasing preference for home-based diagnostics, and accelerated development in wearable health technologies.
Major Manufacturers in the Market
l Philips Respironics
l ResMed
l Fisher & Paykel Healthcare
l BMC Medical
l Nox Medical
l Withings
l Itamar Medical
l Nihon Kohden
l Beurer
l Medtronic
Leading Companies and Innovations
In 2025, companies are rapidly expanding their portfolio of wearable solutions. For example, Samsung recently achieved FDA De Novo clearance for its sleep apnea detection feature integrated into its Galaxy Watch series, while Apple introduced sleep apnea detection across its Apple Watch Series 9, Ultra 2, and Series 10, with a goal of global deployment in over 150 countries. Huxley Medical received FDA clearance for its SANSA chest patch for home diagnostics, and Apnimed launched a ring-shaped pulse oximeter offering extended home-based apnea monitoring.
Product Classifications
· Wristband Type
· Ring Type
· Chest Patch Type
· Head-mounted Type
· Others
Application Segments
· Home Sleep Monitoring
· Hospital and Clinical Diagnosis
· Remote Health Management
· Research Institution Application
· Others
Company Product Models Overview
1. Philips Respironics
§ DreamWear Full Face CPAP Mask
§ DreamStation Auto CPAP with Humidifier
§ Wisp Nasal Mask FitPack
§ Amara View Full Face Mask
§ System One REMstar SE CPAP Machine
2. ResMed
§ AirSense 11 AutoSet CPAP
§ AirMini Travel CPAP
§ AirFit F20 Full Face Mask
§ AirTouch N20 Nasal Mask
§ S9 Elite CPAP Machine
3. Fisher & Paykel Healthcare
§ SleepStyle Auto CPAP
§ Evora Nasal Mask
§ Vitera Full Face Mask
§ Brevida Nasal Pillow Mask
§ HC150 Heated Humidifier
4. Withings
§ Sleep Analyzer Pad
§ ScanWatch Horizon
§ Pulse HR Tracker
§ Body Comp Smart Scale
§ Steel HR Sport Hybrid Smartwatch
5. Itamar Medical
§ WatchPAT ONE Disposable Home Sleep Apnea Test
§ WatchPAT 300 Reusable HSAT Device
§ ZOLL Itamar CloudPAT
6. Nox Medical
§ Nox T3 Portable Sleep Monitor
§ Nox A1 PSG System
§ Noxturnal Sleep Software Suite
7. Nihon Kohden
§ Polysmith Sleep Software
§ EEG-1200 Sleep Diagnostic System
§ QP-160AK PSG Recorder
8. Beurer
§ SL 70 Snore Stopper
§ IH 55 Nebulizer with Sleep Support
§ BY 88 Smart Baby Monitor (sleep analysis)
9. Medtronic
§ Nellcor Portable SpO2 Patient Monitoring System
§ Capnostream 35 Portable Respiratory Monitor
Regional Insights
The market is segmented into key regions: North America, Europe, Asia Pacific, South America, and the Middle East & Africa. North America and Asia-Pacific are expected to be high-growth regions due to healthcare digitization, wearable technology adoption, and growing prevalence of sleep apnea. Europe also shows a strong compound growth rate with increasing government support for remote diagnostics.
Technological and Market Trends
1. Smart Pajamas with Fabric Sensors and Machine Learning
· High Accuracy Monitoring: Researchers at the University of Cambridge have developed washable smart pajamas equipped with printed fabric sensors and a lightweight AI model named SleepNet. These pajamas can identify six different sleep states, including nasal breathing, mouth breathing, snoring, teeth grinding, central sleep apnea, and obstructive sleep apnea, with an accuracy of 98.6%.
· Comfort and Usability: The sensors are designed to detect tiny movements in the skin, even when the pajamas are worn loosely around the neck and chest. This design ensures comfort without compromising data accuracy.
· Wireless Data Transmission: The smart pajamas can wirelessly transmit data to a smartphone or computer, facilitating long-term health monitoring without the need for hospital visits.
· Energy Efficiency: The energy-efficient sensors require only a handful of examples of sleep patterns to identify the difference between regular and disordered sleep, making them practical for extended use.
2. Integration of Millimeter-Wave Radar and Pulse Oximetry
· ROSA System Development: A novel method named ROSA (Radar and Oximeter Sleep Apnea) has been developed, combining millimeter-wave radar and pulse oximeter data to diagnose obstructive sleep apnea-hypopnea syndrome (OSAHS). This method aims to achieve diagnostic performance comparable to the gold-standard polysomnography (PSG) with significantly reduced monitoring burden.
· High Diagnostic Accuracy: In a study involving over 800 hours of overnight recordings from 100 subjects, ROSA demonstrated high agreement (Intraclass Correlation Coefficient = 0.9870) on the apnea-hypopnea index (AHI) between ROSA and PSG. The system exhibited over 90% accuracy across various AHI diagnostic thresholds.
· Non-Invasive Monitoring: The use of millimeter-wave radar allows for contactless monitoring of physiological characteristics such as human respiration and heartbeat, providing a comfortable alternative to traditional methods.
3. Market Implications and Future Directions
· Shift Towards Home-Based Monitoring: The development of comfortable, at-home sleep-monitoring solutions like smart pajamas and ROSA aligns with a broader trend of moving away from cumbersome clinical equipment towards more accessible, reliable home-monitoring tools.
· Potential for Broader Health Applications: Researchers aim to adapt the technology used in smart pajamas for other health monitoring uses, including baby monitoring and tracking additional respiratory or neurological conditions.
· Economic Impact: With sleep disorders contributing to significant health risks and economic burdens, the introduction of these innovative monitoring tools is seen as crucial for improving public health outcomes and reducing healthcare costs.
Market Dynamics
Key market drivers include rising global awareness about sleep disorders, policy support for home healthcare, and user preference for minimally invasive solutions. However, the market also faces uncertainties due to tariff policy shifts in the U.S. in 2025, which could influence supply chains and component costs. Companies are responding with diversified sourcing strategies and increased regional partnerships.
Conclusion
With robust demand, continuous innovation, and increasing adoption in home and clinical settings, the wearable sleep apnea detector market is poised for dynamic growth through 2031. Industry leaders are expected to compete on innovation, accuracy, and comfort, while regional players expand through strategic collaborations and regulatory clearances.
Get free Sample report:
Global Wearable Sleep Apnea Detector Market Research Report 2025
https://www.qyresearch.com/reports/4742057/wearable-sleep-apnea-detector
Wearable Sleep Apnea Detector - Global Market Share and Ranking, Overall Sales and Demand Forecast 2025-2031
https://www.qyresearch.com/reports/4742056/wearable-sleep-apnea-detector
Global Wearable Sleep Apnea Detector Sales Market Report, Competitive Analysis and Regional Opportunities 2025-2031
https://www.qyresearch.com/reports/4742055/wearable-sleep-apnea-detector
Global Wearable Sleep Apnea Detector Market Insights, Forecast to 2031
https://www.qyresearch.com/reports/4742054/wearable-sleep-apnea-detector
About Us
QYResearch founded in California, USA in 2007. It is a leading global market research and consulting company. With over 18 years’ experience and professional research team in various cities over the world QY Research focuses on management consulting, database and seminar services, IPO consulting, industry chain research and customized research to help our clients in providing non-linear revenue model and make them successful. We are globally recognized for our expansive portfolio of services, good corporate citizenship, and our strong commitment to sustainability. Up to now, we have cooperated with more than 66,000 clients across five continents. Let’s work closely with you and build a bold and better future.
Contact Us:
If you have any queries regarding this report or if you would like further information, please contact us:
QY Research Inc.
Add: 17890 Castleton Street Suite 369 City of Industry CA 91748 United States
EN: https://www.qyresearch.com
Email: global@qyresearch.com
Tel: 001-626-842-1666(US)
JP: https://www.qyresearch.co.jp
More Posts



















Report This Post
Please complete the following requested information to flag this post and report abuse, or offensive content. Your report will be reviewed within 24 hours. We will take appropriate action as described in Findit terms of use.


